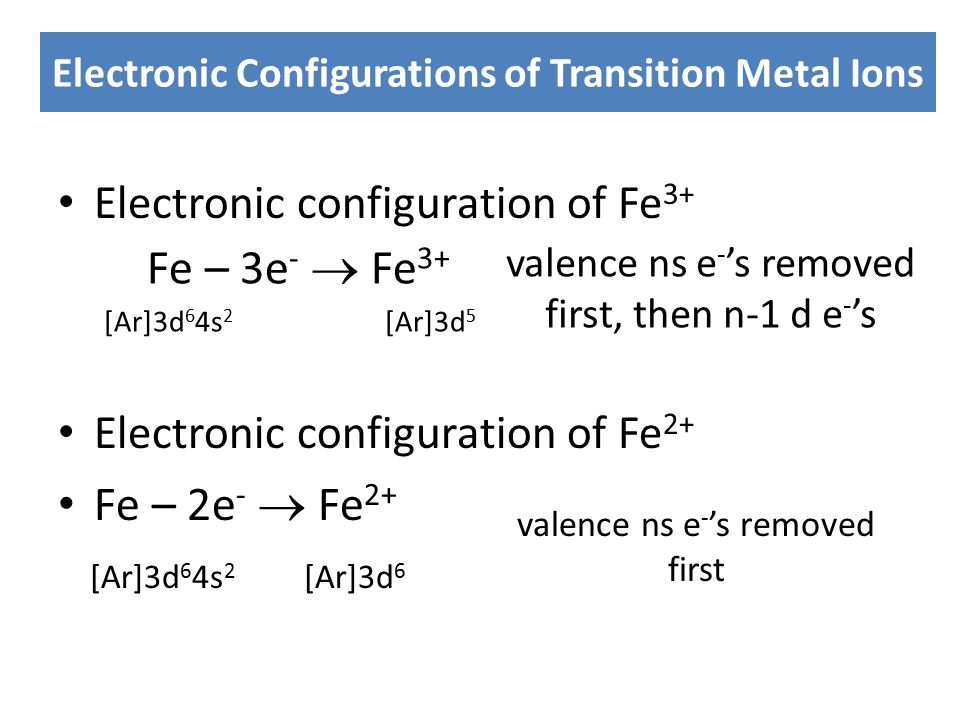
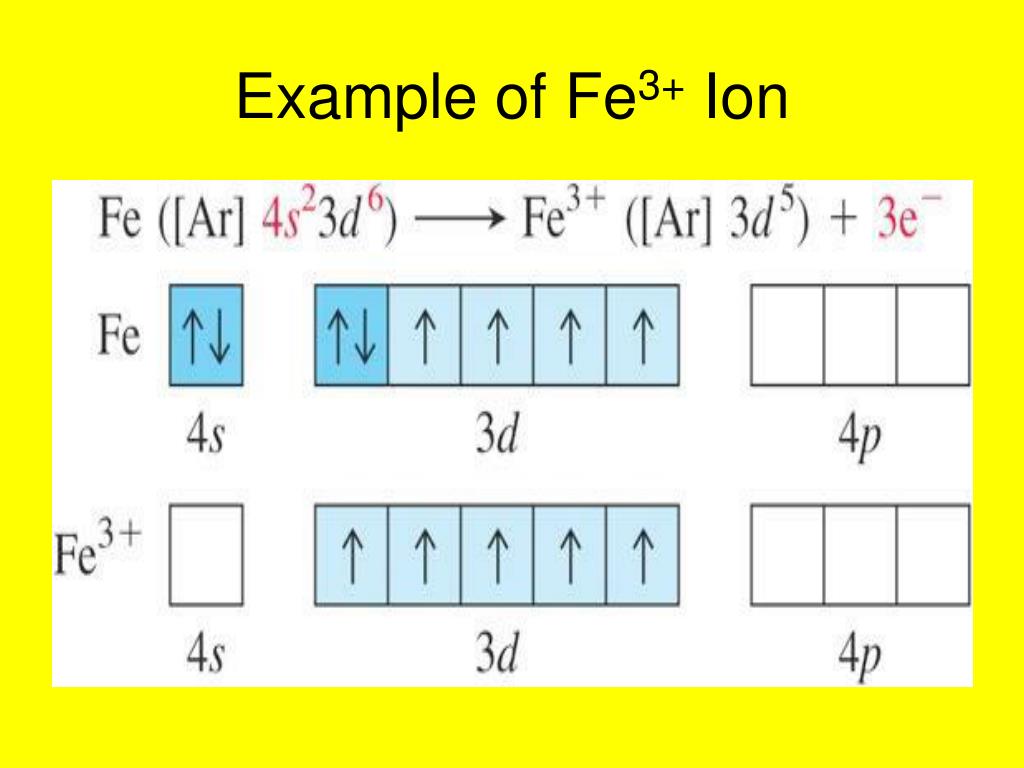
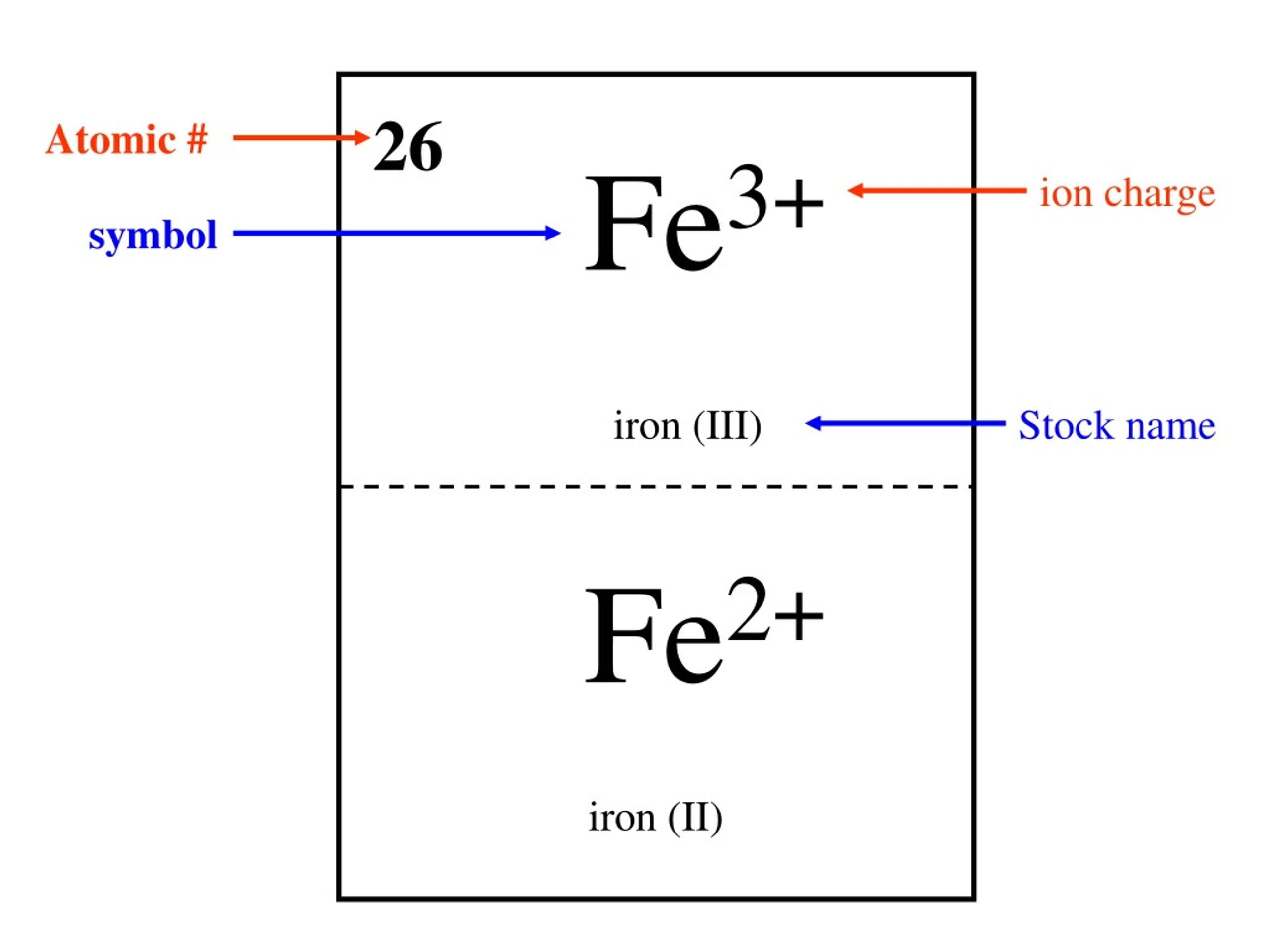
The Cation Fe3+ Is Formed When - When writing the formula of a compound, the cation is listed before the anion. A cation has more protons than electrons, consequently giving it a net positive charge. For example, in nacl, the sodium atom acts as the. For example, na is the cation and cl is the. Summary cations are formed by the loss of one or two electrons from an element. The chemical formula of a compound is always written with the cation first, followed by the anion. A cation is something that moves down (greek: Groups 1 and 2 elements form cations. For a cation to form, one or more. Κάτω, kato, meaning down) and an anion is something that moves up (greek:
Groups 1 and 2 elements form cations. For a cation to form, one or more. When writing the formula of a compound, the cation is listed before the anion. The chemical formula of a compound is always written with the cation first, followed by the anion. Κάτω, kato, meaning down) and an anion is something that moves up (greek: For example, na is the cation and cl is the. For example, in nacl, the sodium atom acts as the. A cation has more protons than electrons, consequently giving it a net positive charge. Summary cations are formed by the loss of one or two electrons from an element. A cation is something that moves down (greek:
The chemical formula of a compound is always written with the cation first, followed by the anion. When writing the formula of a compound, the cation is listed before the anion. For a cation to form, one or more. A cation has more protons than electrons, consequently giving it a net positive charge. For example, na is the cation and cl is the. A cation is something that moves down (greek: Κάτω, kato, meaning down) and an anion is something that moves up (greek: Summary cations are formed by the loss of one or two electrons from an element. For example, in nacl, the sodium atom acts as the. Groups 1 and 2 elements form cations.
Lecture 17. The dBlock Elements. General properties ppt download
For example, na is the cation and cl is the. Summary cations are formed by the loss of one or two electrons from an element. For a cation to form, one or more. The chemical formula of a compound is always written with the cation first, followed by the anion. When writing the formula of a compound, the cation is.
Nomenclature and writing chemical equations ppt download
A cation has more protons than electrons, consequently giving it a net positive charge. Summary cations are formed by the loss of one or two electrons from an element. When writing the formula of a compound, the cation is listed before the anion. A cation is something that moves down (greek: For example, na is the cation and cl is.
PPT Wave Nature of Light PowerPoint Presentation, free download ID
Summary cations are formed by the loss of one or two electrons from an element. When writing the formula of a compound, the cation is listed before the anion. For example, na is the cation and cl is the. The chemical formula of a compound is always written with the cation first, followed by the anion. For a cation to.
Chapter 8 Periodic Relationships Among the Elements ppt download
Groups 1 and 2 elements form cations. When writing the formula of a compound, the cation is listed before the anion. For example, na is the cation and cl is the. A cation is something that moves down (greek: Κάτω, kato, meaning down) and an anion is something that moves up (greek:
Chapter 7 Ionic & Covalent Bonds. ppt download
A cation is something that moves down (greek: For example, in nacl, the sodium atom acts as the. Κάτω, kato, meaning down) and an anion is something that moves up (greek: A cation has more protons than electrons, consequently giving it a net positive charge. Groups 1 and 2 elements form cations.
Chemistry I Notes Unit 3 Chapters ppt download
For a cation to form, one or more. For example, in nacl, the sodium atom acts as the. Groups 1 and 2 elements form cations. When writing the formula of a compound, the cation is listed before the anion. Summary cations are formed by the loss of one or two electrons from an element.
PPT Chemical Nomenclature (Naming Compounds) PowerPoint Presentation
A cation is something that moves down (greek: When writing the formula of a compound, the cation is listed before the anion. A cation has more protons than electrons, consequently giving it a net positive charge. For a cation to form, one or more. The chemical formula of a compound is always written with the cation first, followed by the.
Nomenclature of Compounds ppt download
For a cation to form, one or more. Κάτω, kato, meaning down) and an anion is something that moves up (greek: For example, in nacl, the sodium atom acts as the. Summary cations are formed by the loss of one or two electrons from an element. When writing the formula of a compound, the cation is listed before the anion.
Bonding General Concepts ppt download
For example, na is the cation and cl is the. For example, in nacl, the sodium atom acts as the. A cation is something that moves down (greek: Κάτω, kato, meaning down) and an anion is something that moves up (greek: Groups 1 and 2 elements form cations.
Periodicity. ppt download
A cation is something that moves down (greek: Groups 1 and 2 elements form cations. For example, na is the cation and cl is the. Κάτω, kato, meaning down) and an anion is something that moves up (greek: Summary cations are formed by the loss of one or two electrons from an element.
For Example, Na Is The Cation And Cl Is The.
A cation is something that moves down (greek: For example, in nacl, the sodium atom acts as the. Summary cations are formed by the loss of one or two electrons from an element. Groups 1 and 2 elements form cations.
The Chemical Formula Of A Compound Is Always Written With The Cation First, Followed By The Anion.
A cation has more protons than electrons, consequently giving it a net positive charge. For a cation to form, one or more. When writing the formula of a compound, the cation is listed before the anion. Κάτω, kato, meaning down) and an anion is something that moves up (greek: