Dimethyl Sulfoxide Safety Data Sheet - How does the reaction proceed?? I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile. I understand that the trans isomer doesn't possess. I came across the reaction of dimethyl sulfate with water to yield methanol. In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. Is it oxygen lone pair attacks on sulphur which. Dimethyl sulphate breaks the oh bond and forms anisole. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups.
I came across the reaction of dimethyl sulfate with water to yield methanol. In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups. Is it oxygen lone pair attacks on sulphur which. How does the reaction proceed?? Dimethyl sulphate breaks the oh bond and forms anisole. I understand that the trans isomer doesn't possess. I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile.
I came across the reaction of dimethyl sulfate with water to yield methanol. How does the reaction proceed?? I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile. Is it oxygen lone pair attacks on sulphur which. I understand that the trans isomer doesn't possess. Dimethyl sulphate breaks the oh bond and forms anisole. In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups.
Dimethyl sulfoxide, properties, uses and safety protection Shenyang
How does the reaction proceed?? In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. Dimethyl sulphate breaks the oh bond and forms anisole. I came across the reaction of dimethyl sulfate with water to yield methanol. Is it oxygen lone pair attacks on sulphur which.
Dimethyl Sulfoxide Baijin
How does the reaction proceed?? I came across the reaction of dimethyl sulfate with water to yield methanol. Is it oxygen lone pair attacks on sulphur which. Dimethyl sulphate breaks the oh bond and forms anisole. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups.
DMSO Msds PDF Dimethyl Sulfoxide Toxicity
Dimethyl sulphate breaks the oh bond and forms anisole. Is it oxygen lone pair attacks on sulphur which. How does the reaction proceed?? I came across the reaction of dimethyl sulfate with water to yield methanol. I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile.
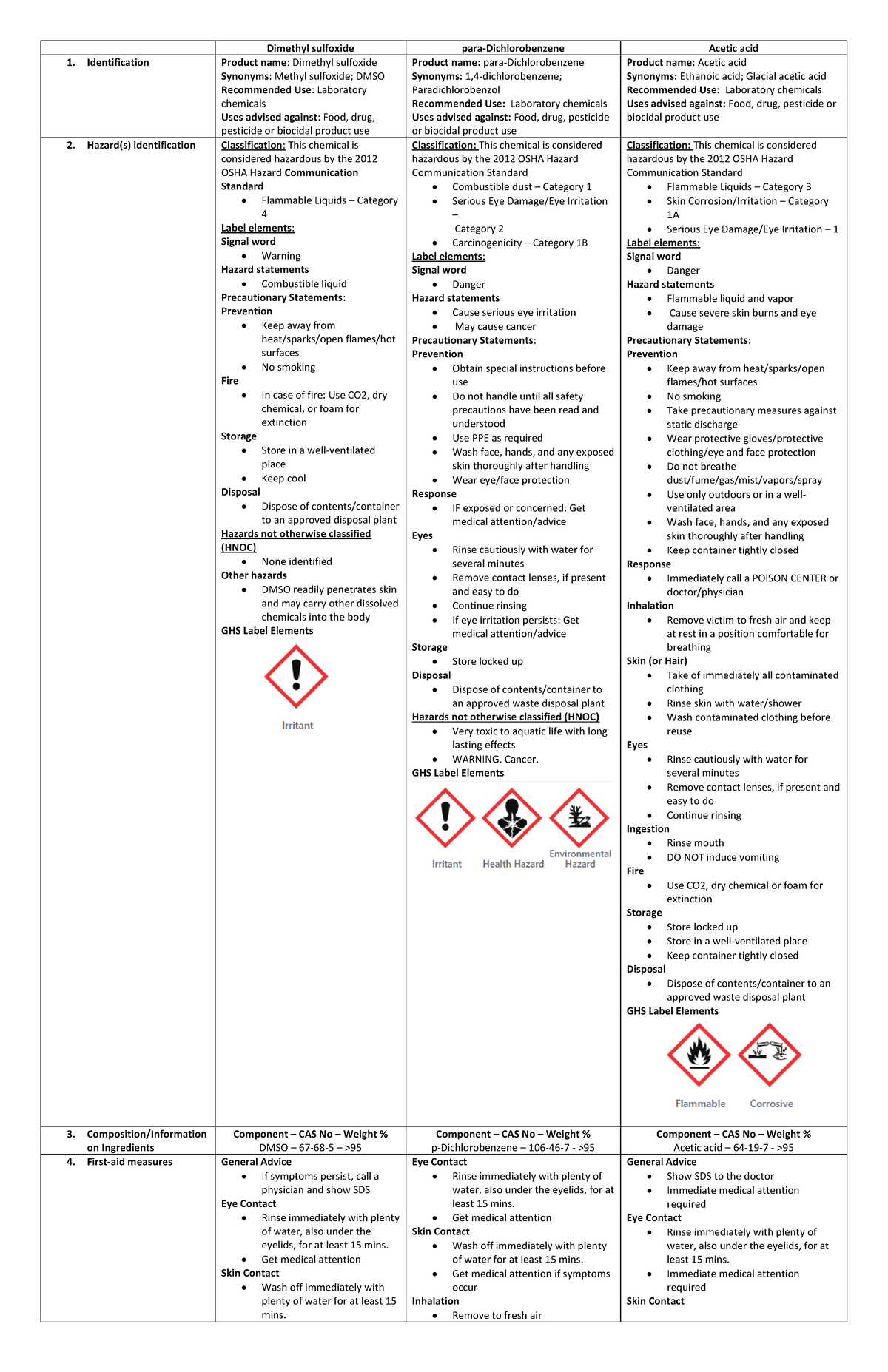
Safety Data Sheets (DMSO, pDichlorobenzene, Acetic acid) Dimethyl
I understand that the trans isomer doesn't possess. How does the reaction proceed?? I came across the reaction of dimethyl sulfate with water to yield methanol. I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups.
Dimethyl Sulfoxide Baijin
Dimethyl sulphate breaks the oh bond and forms anisole. In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. I understand that the trans isomer doesn't possess. I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile. 1 i'm teaching myself organic chemistry and am struggling with differentiating between.
Dimethyl Disulfide Safety Data Sheet Download Free PDF Toxicity
In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. I came across the reaction of dimethyl sulfate with water to yield methanol. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups. I understand that the trans isomer doesn't possess. Dimethyl sulphate breaks.
Dimethyl Disulfide Safety Data Sheet PDF Dangerous Goods
I understand that the trans isomer doesn't possess. I came across the reaction of dimethyl sulfate with water to yield methanol. I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups. In cell culture, dimethylsulfoxide (dmso) is added to prevent the.
(PDF) Structural parameters of dimethyl sulfoxide, DMSO, at 100 K
How does the reaction proceed?? 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups. In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. I came across the reaction of dimethyl sulfate with water to yield methanol. Dimethyl sulphate breaks the oh bond.
(PDF) MATERIAL SAFETY DATA SHEET DMSO … MSDS/DMSO MSDS Liquid
I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups. Dimethyl sulphate breaks the oh bond and forms anisole. In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. I understand that.
Fillable Online Dimethyl sulfoxide Safety Data Sheet Fax Email Print
In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. Dimethyl sulphate breaks the oh bond and forms anisole. 1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups. I understand that the trans isomer doesn't possess. I came across the reaction of dimethyl.
How Does The Reaction Proceed??
Dimethyl sulphate breaks the oh bond and forms anisole. Is it oxygen lone pair attacks on sulphur which. In cell culture, dimethylsulfoxide (dmso) is added to prevent the formation of ice crystals which may lyse the cells. I was curious how that works as $\\ce{h2o}$ isn't a strong nucleophile.
I Understand That The Trans Isomer Doesn't Possess.
1 i'm teaching myself organic chemistry and am struggling with differentiating between dimethyl and ethyl substituent groups. I came across the reaction of dimethyl sulfate with water to yield methanol.



.png)




