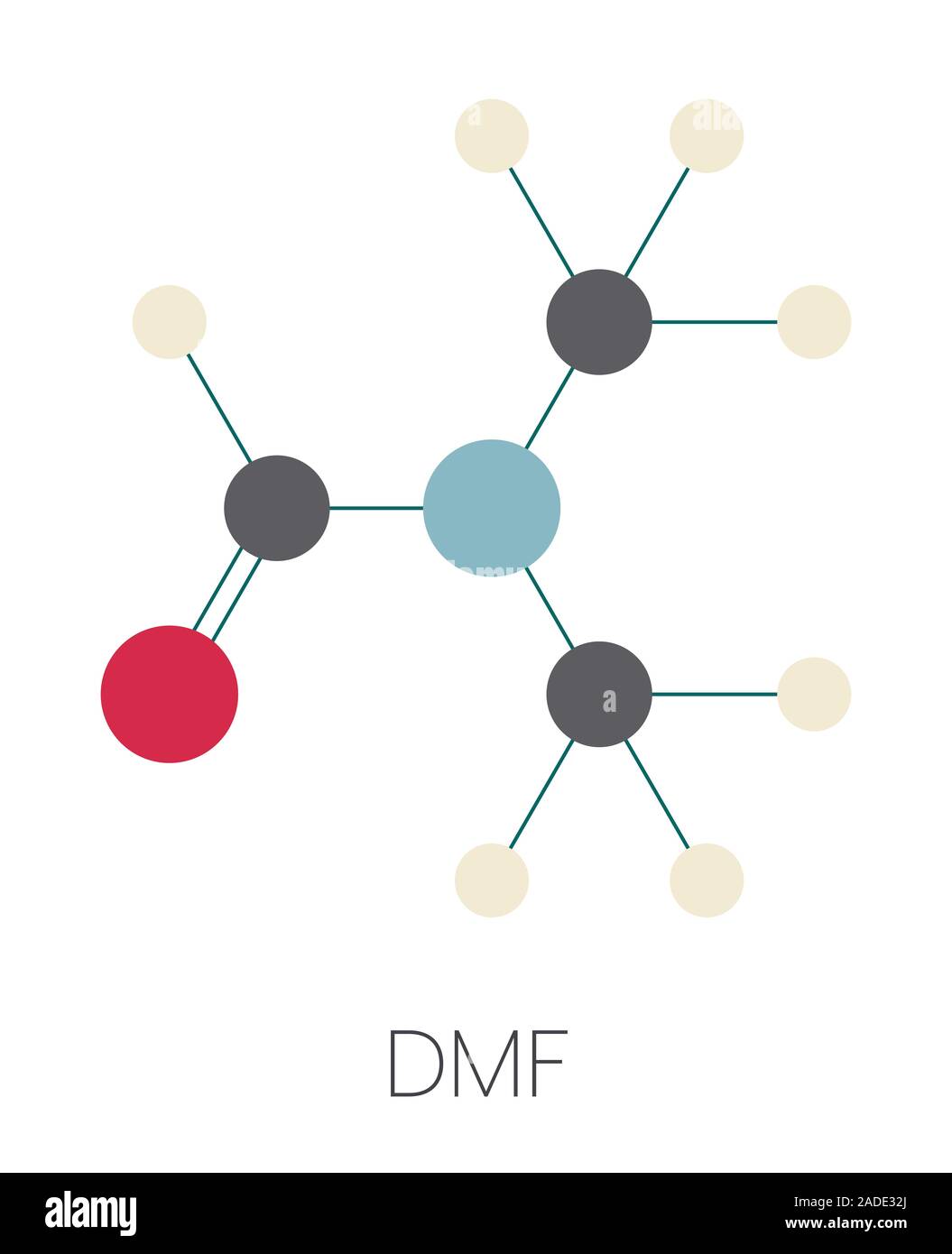
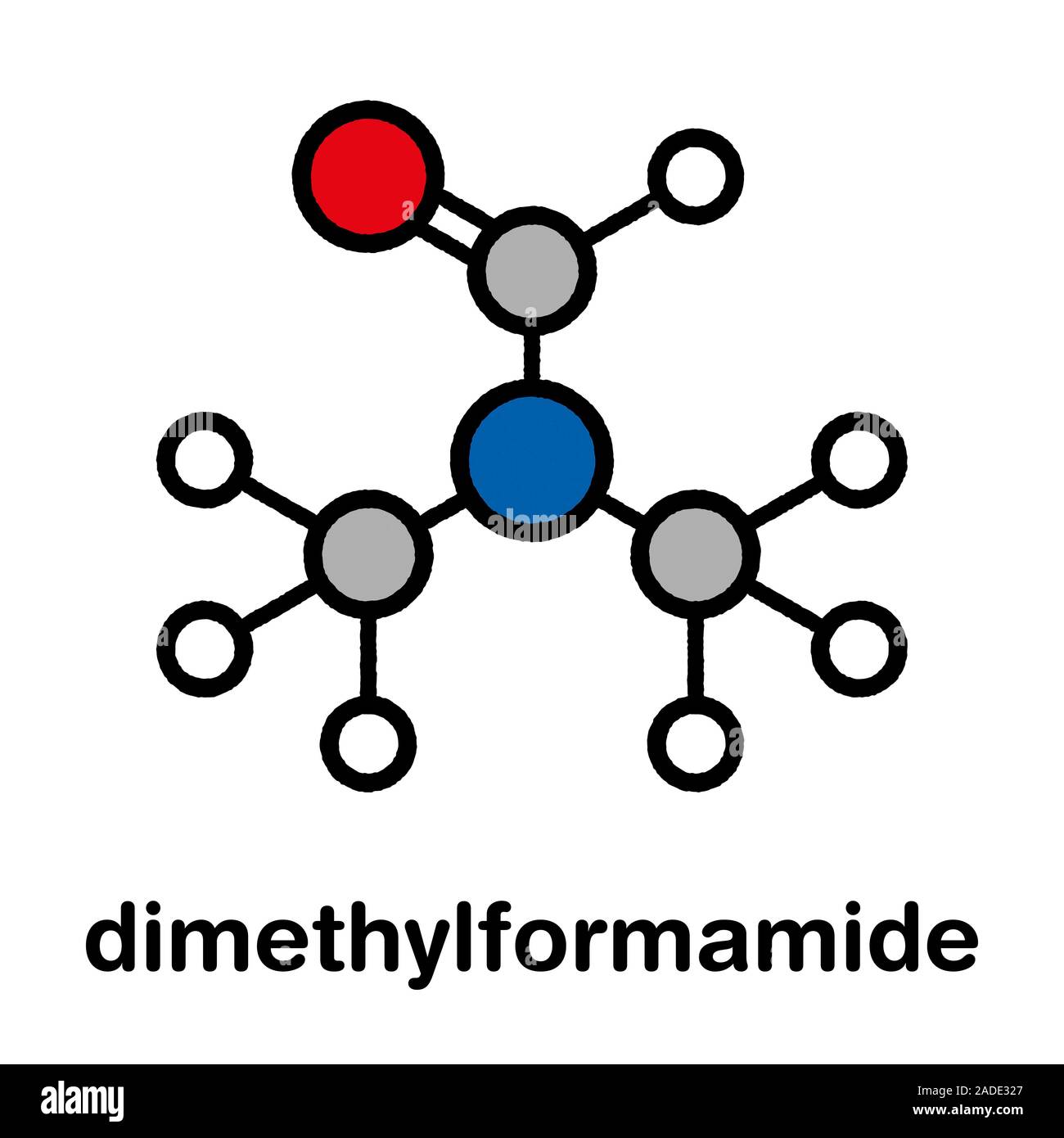
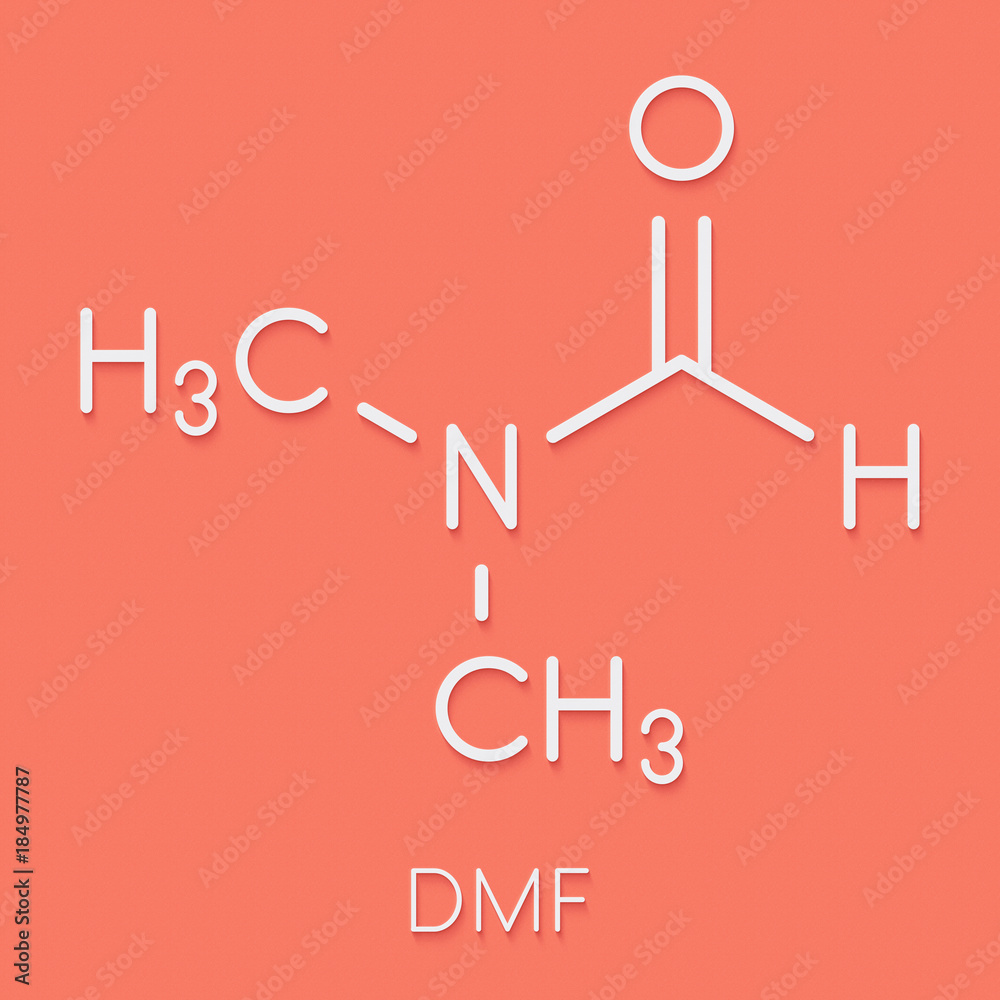
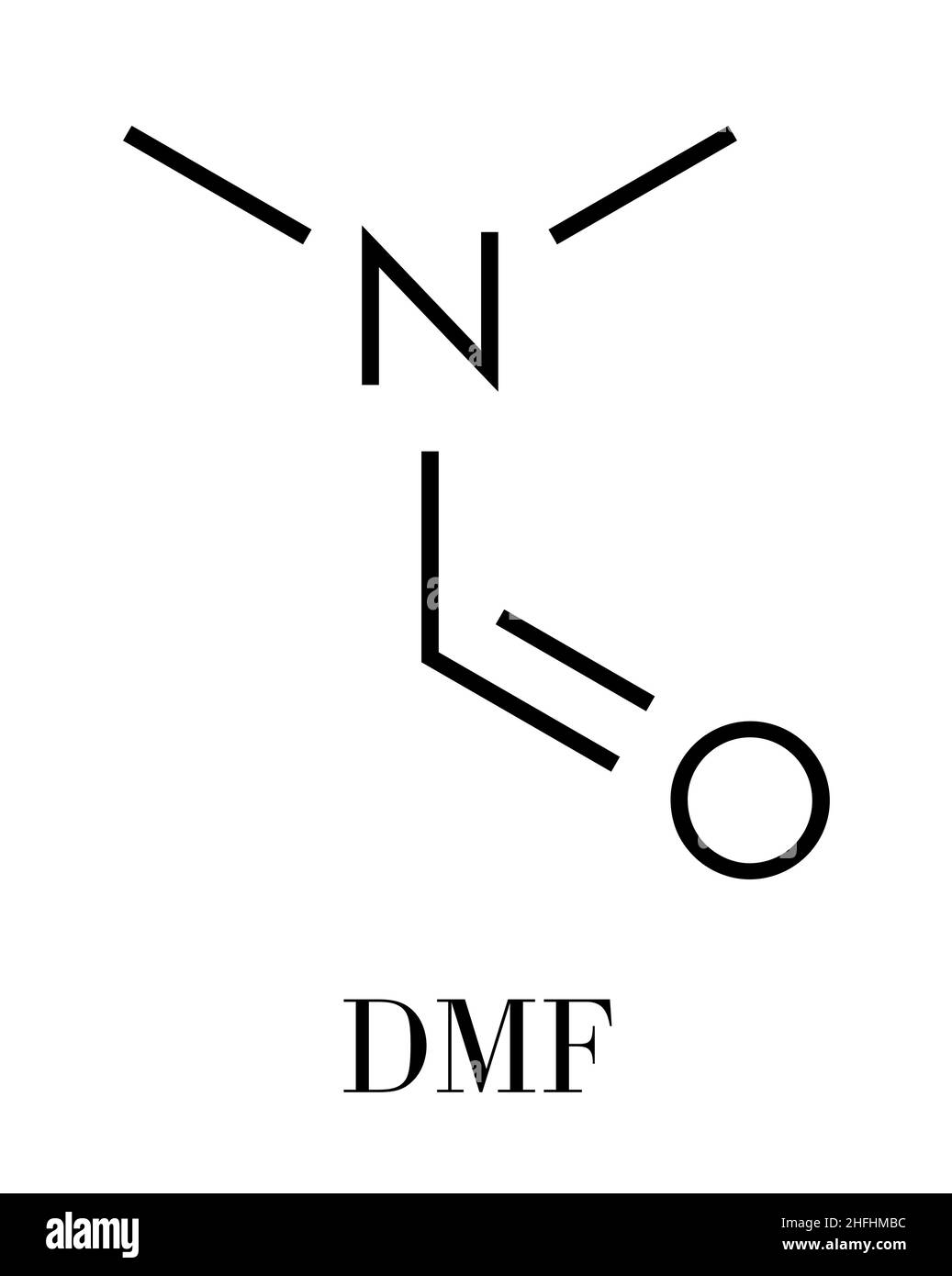
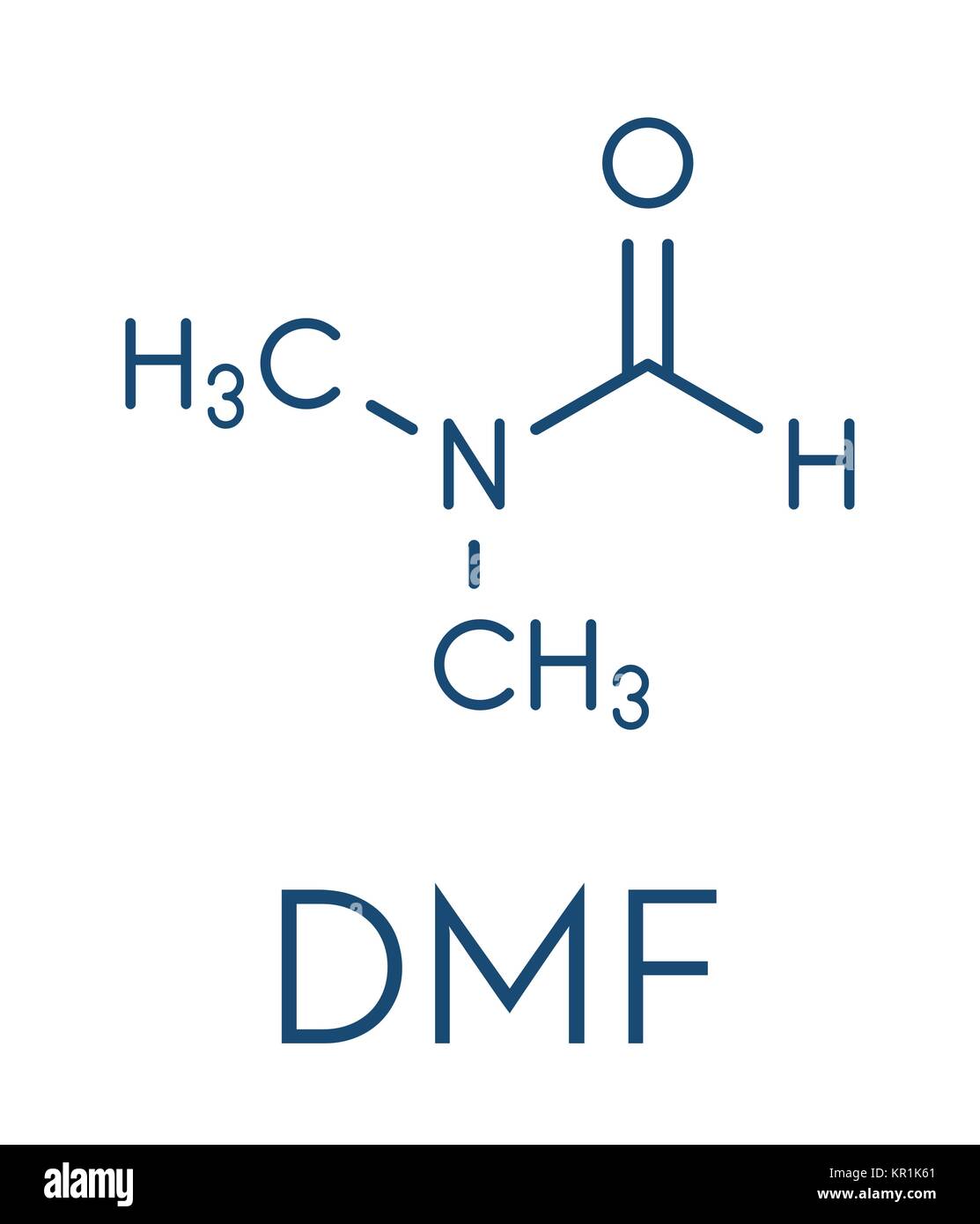
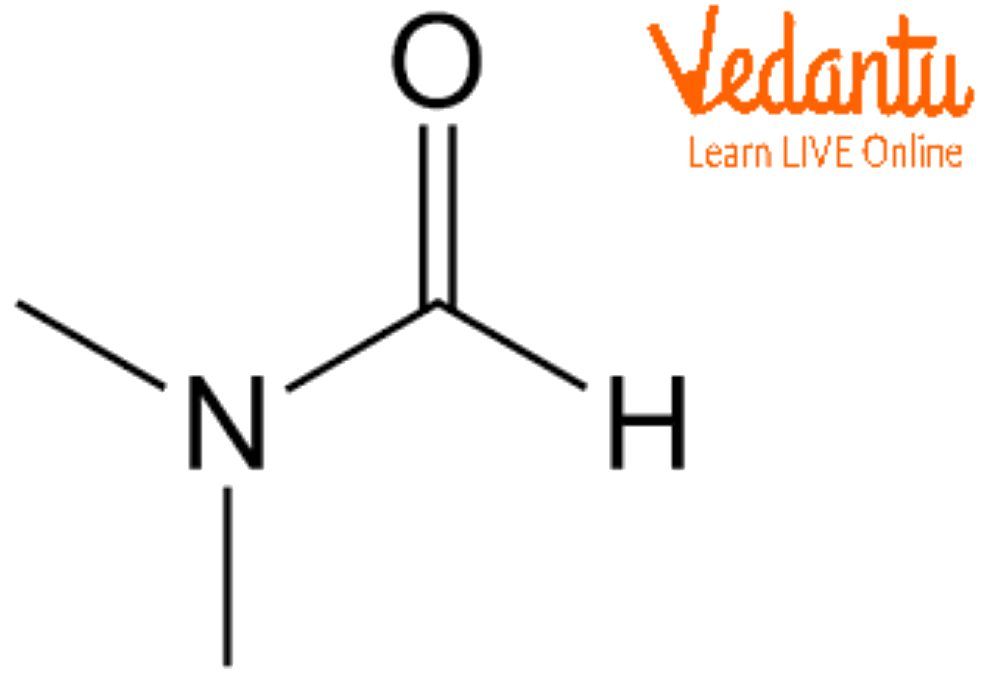
Dmf Full Form - Dmf is a useful solvent employed for the isolation of. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Its structure is hc (=o)−n (−ch 3)2. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is also used as a reagent in some reactions.
Dmf is also used as a reagent in some reactions. Its structure is hc (=o)−n (−ch 3)2. Dmf is a useful solvent employed for the isolation of. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c).
Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Dmf is a useful solvent employed for the isolation of. Its structure is hc (=o)−n (−ch 3)2. Dmf is also used as a reagent in some reactions. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c).
Dimethylformamide (DMF) chemical solvent molecule. Stylized skeletal
Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Its structure is hc (=o)−n (−ch 3)2. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is also used as a reagent in some reactions. Dmf is a useful solvent employed for the isolation of.
Molecular structure of dimethyl formamide (DMF). Download Scientific
Dmf is a useful solvent employed for the isolation of. Its structure is hc (=o)−n (−ch 3)2. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is also used as a reagent in some reactions.
Dimethylformamide (DMF) chemical solvent molecule. Stylized skeletal
Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is also used as a reagent in some reactions. Dmf is a useful solvent employed for the isolation of. Its structure is hc (=o)−n (−ch 3)2. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl.
Dimethylformamide Structure
Its structure is hc (=o)−n (−ch 3)2. Dmf is also used as a reagent in some reactions. Dmf is a useful solvent employed for the isolation of. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c).
Proposed chemistry of NDMA formation from DMF during sartan production
Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Its structure is hc (=o)−n (−ch 3)2. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is also used as a reagent in some reactions. Dmf is a useful solvent employed for the isolation of.
Dimethylformamide DMF Chemical Solvent Skeletal Stock , 52 OFF
Dmf is also used as a reagent in some reactions. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is a useful solvent employed for the isolation of. Its structure is hc (=o)−n (−ch 3)2.
Dimethylformamide Structure
Its structure is hc (=o)−n (−ch 3)2. Dmf is a useful solvent employed for the isolation of. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is also used as a reagent in some reactions.
Difference Between DMF And DMSO Compare The Difference, 42 OFF
Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Dmf is a useful solvent employed for the isolation of. Dmf is also used as a reagent in some reactions. Its structure is hc (=o)−n (−ch 3)2.
Dimethylformamide Structure
Its structure is hc (=o)−n (−ch 3)2. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is also used as a reagent in some reactions. Dmf is a useful solvent employed for the isolation of. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl.
DIMETHYL FORMAMIDE DMF at Rs 80 Laboratory Chemicals in Dombivli ID
Dmf is also used as a reagent in some reactions. Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl. Its structure is hc (=o)−n (−ch 3)2. Dmf is a useful solvent employed for the isolation of.
Its Structure Is Hc (=O)−N (−Ch 3)2.
Dimethylformamide (dmf) is a polar aprotic solvent with a high boiling point (153 c). Dmf is a useful solvent employed for the isolation of. Dmf is also used as a reagent in some reactions. Commonly abbreviated as dmf (although this initialism is sometimes used for dimethylfuran, or dimethyl.