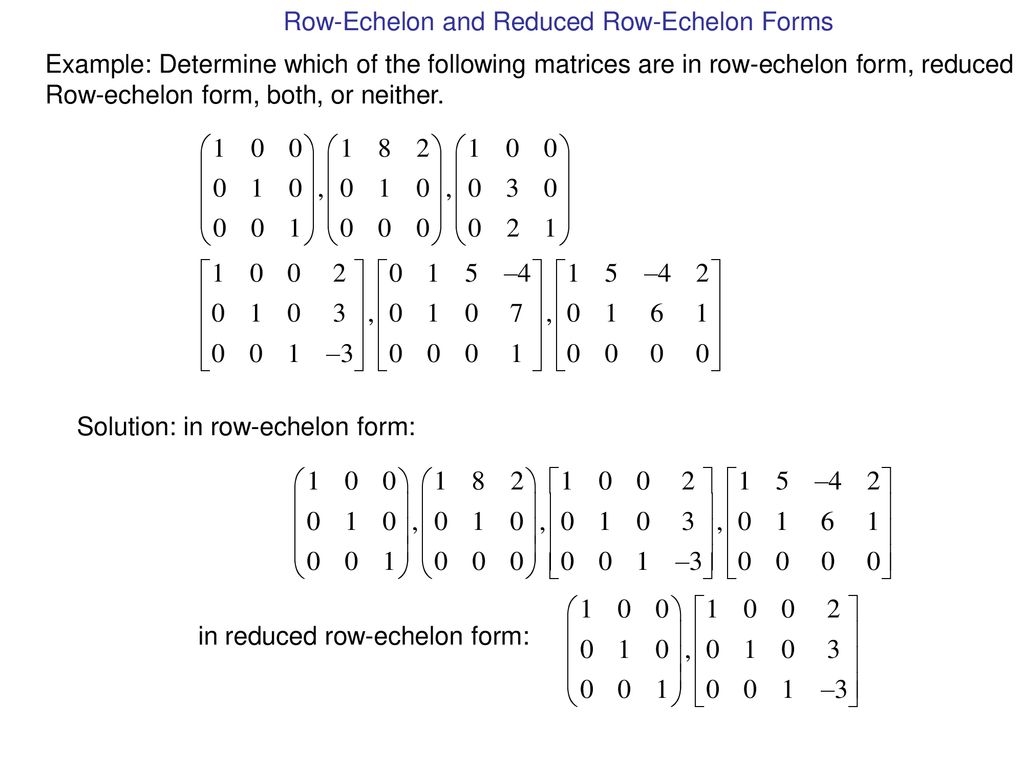
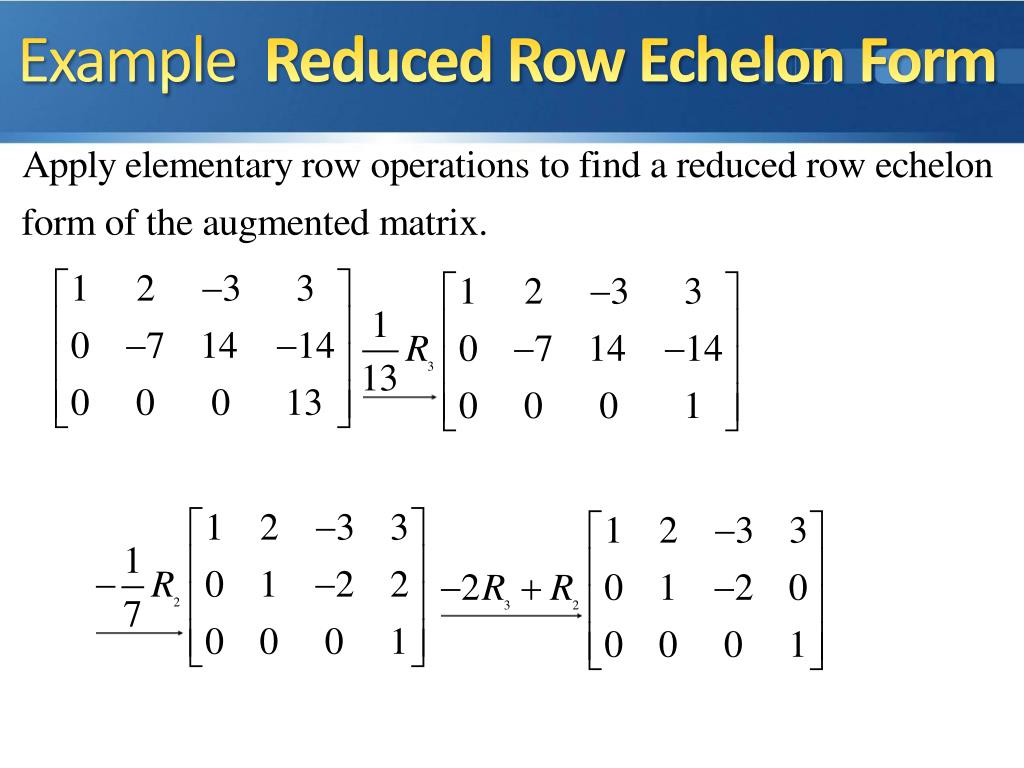
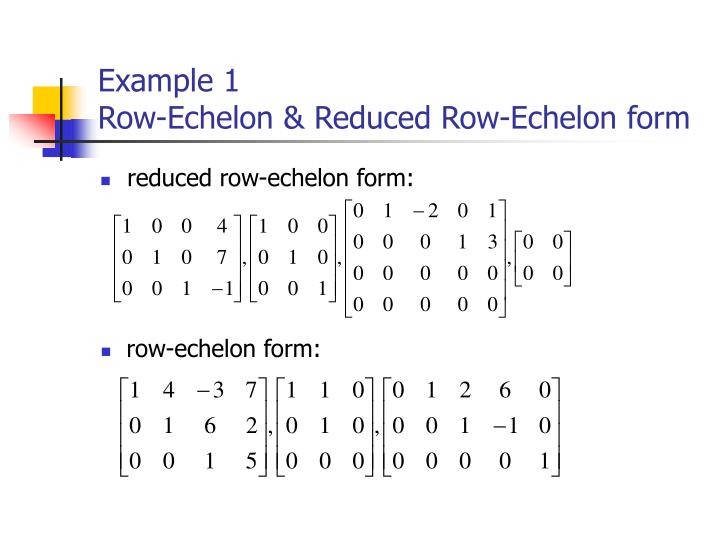
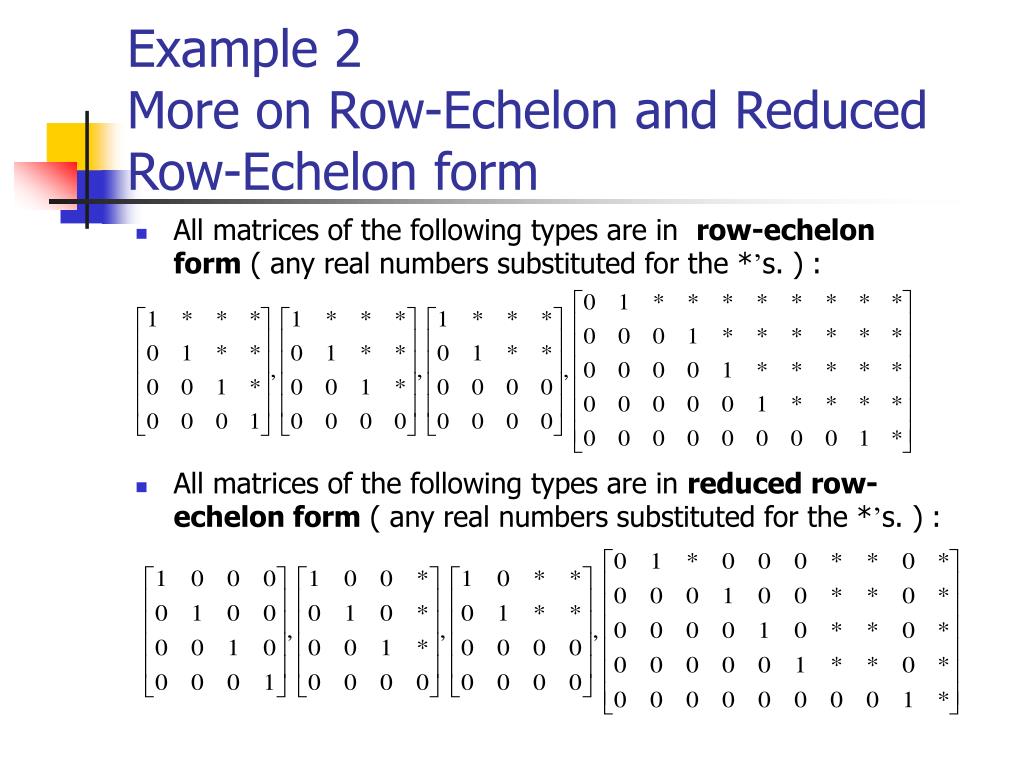
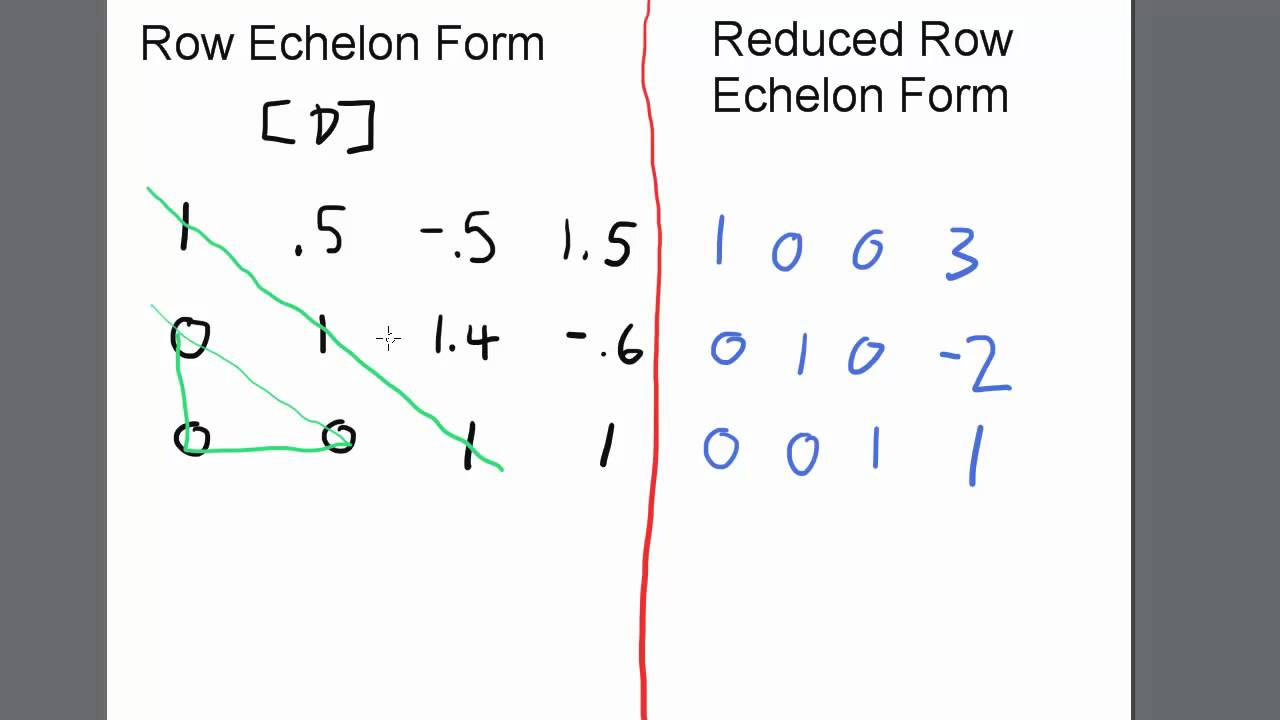
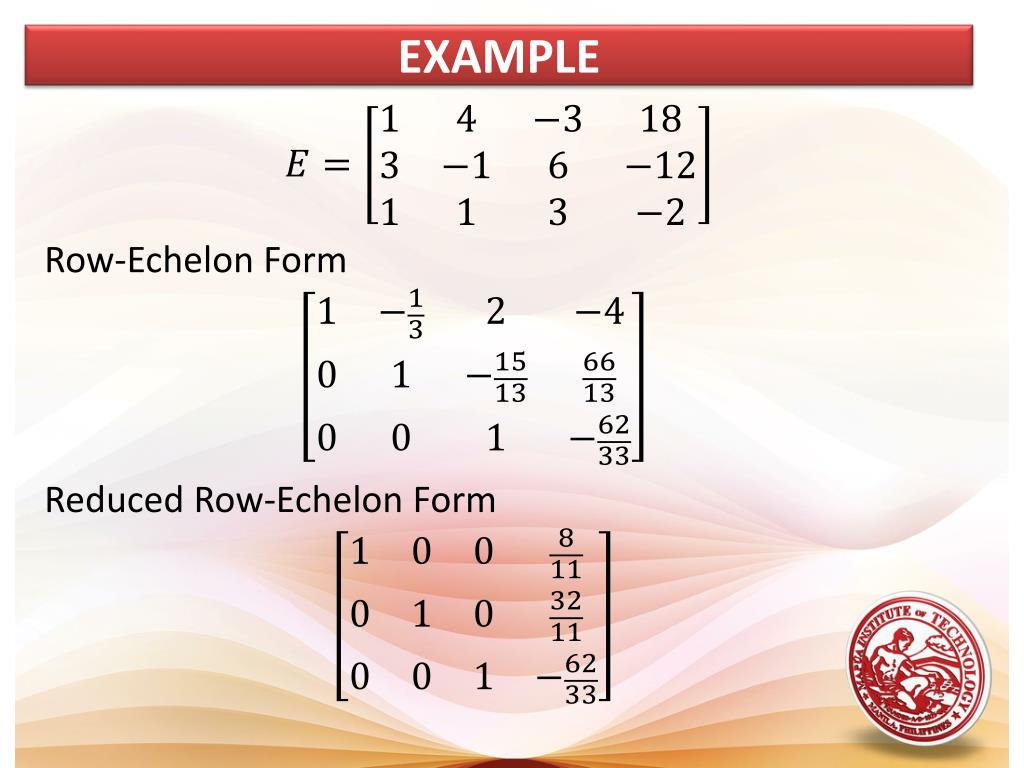
Echelon Form And Reduced Echelon Form Examples - The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. I know that the first shell can hold up to 2, the second and third can hold up to 8, and the fourth 18. But what about after that? The first shell can only hold 2 electrons; In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². The second shell can hold 8, the third can hold 18, the fourth can hold 32, and. Other shells have different maxima: As it turns out, each shell's maximum is a noble gas configuration. Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s.
I know that the first shell can hold up to 2, the second and third can hold up to 8, and the fourth 18. But what about after that? The first shell can only hold 2 electrons; Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s. The second shell can hold 8, the third can hold 18, the fourth can hold 32, and. The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². Other shells have different maxima: As it turns out, each shell's maximum is a noble gas configuration.
The first shell can only hold 2 electrons; But what about after that? The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. The second shell can hold 8, the third can hold 18, the fourth can hold 32, and. I know that the first shell can hold up to 2, the second and third can hold up to 8, and the fourth 18. Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s. Other shells have different maxima: In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². As it turns out, each shell's maximum is a noble gas configuration.
Chapter 1 Systems of Linear Equations and Matrices ppt download
The second shell can hold 8, the third can hold 18, the fourth can hold 32, and. The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. In my textbook, it says that the maximum number of electrons that can fit in any.
PPT Multivariate Linear Systems and Row Operations PowerPoint
In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². The second shell can hold 8, the third can hold 18, the fourth can hold 32, and. I know that the first shell can hold up to 2, the second and third can hold up to 8, and.
Echelon Form Solution 28493 Calculating The Reduced Row Echelon Form
But what about after that? As it turns out, each shell's maximum is a noble gas configuration. Other shells have different maxima: The first shell can only hold 2 electrons; In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n².
PPT Elementary Linear Algebra PowerPoint Presentation ID3029972
The second shell can hold 8, the third can hold 18, the fourth can hold 32, and. The first shell can only hold 2 electrons; As it turns out, each shell's maximum is a noble gas configuration. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². But.
PPT Elementary Linear Algebra PowerPoint Presentation, free download
In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n². Other shells have different maxima: The second shell can hold 8, the third can hold 18, the fourth can hold 32, and. Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s. The.
Row Echelon Form Vs Reduced Row Echelon Form, 57 OFF
As it turns out, each shell's maximum is a noble gas configuration. Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s. The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. The first shell can only hold.
PPT ROWECHELON FORM AND REDUCED ROWECHELON FORM PowerPoint
Other shells have different maxima: The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. But what about after that? As it turns out, each shell's maximum is a noble gas configuration. I know that the first shell can hold up to 2,.
Linear Equations in Linear Algebra ppt download
I know that the first shell can hold up to 2, the second and third can hold up to 8, and the fourth 18. Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s. The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has.
Linear Equations in Linear Algebra ppt download
Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s. As it turns out, each shell's maximum is a noble gas configuration. The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. I know that the first shell.
Linear Algebra Row Echelon Form and Reduced Row Echelon Form PPTX
But what about after that? The first shell can only hold 2 electrons; The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given.
But What About After That?
Other shells have different maxima: I know that the first shell can hold up to 2, the second and third can hold up to 8, and the fourth 18. As it turns out, each shell's maximum is a noble gas configuration. In my textbook, it says that the maximum number of electrons that can fit in any given shell is given by 2n².
The Second Shell Can Hold 8, The Third Can Hold 18, The Fourth Can Hold 32, And.
Helium has only 1s filled, neon has 1s, 2s, 2p filled, then argon has 1s. The p orbital can hold a total of 6 (4l+2 where l = 1) electrons and so the boron atom has the following configuration (1s)2 (2s)2 (2p)1. The first shell can only hold 2 electrons;